
This is your ultimate guide to deep cycle battery winter storage.
In it, you’ll learn:
The effects of temperature on batteries
Proper deep cycle battery storage practices
Maintaining deep cycle batteries over the winter
And lots more!
Let’s dive in!
Deep cycle batteries rely on an electrochemical process between lead plates and an electrolyte to produce electrical energy.
Let’s expand on this.
Typically, a flooded lead-acid battery consists of:
Lead oxide plate: This is the positive plate and is covered with a paste of lead dioxide
Sponge lead: This is the negative plate
Electrolyte battery fluid: This is a composition of sulfuric acid (usually 30% to 50%) and water
How do these components interact to produce electricity?
Firstly, the two lead plates are immersed in the electrolyte solution.
And electrons are produced when the plates and solution react chemically.
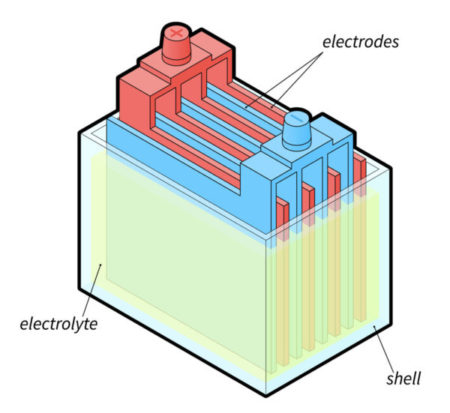
These electrons then flow into the device connected to the battery, powering it.
Once the battery is discharged, charging results in the opposite process happening.
That is, electricity from the charger flows into the battery and is converted into chemical energy.
Now that you know how a lead-acid battery works, it’ll be easier to understand how cold affects them.
Overall, cold weather affects lead-acid batteries in 4 important ways:
The electrolyte can freeze
The battery can lose capacity
The battery will require higher voltages to charge
The battery has a lower self-discharge rate
Let’s go through each aspect in more detail.
1. The Electrolyte Solution Can Freeze
Does battery acid freeze?
Yes, it can.
Here’s how...
When a battery is fully charged, the electrolyte solution (sulfuric acid and water) is evenly mixed.
In that case, the lead-acid battery freezing point is around -76° F.
Hence, a fully charged battery is less likely to freeze in cold temperatures.
Thus, since water has a freezing point of 32° F, the battery is liable to freeze at a relatively higher temperature than when charged.
So, it’s important to keep your batteries fully charged in very cold weather.
That way, you’ll have a properly balanced electrolyte.
And that means the electrolyte will be more resistant to freezing.
However, when a battery is discharged, the sulfuric acid from the electrolyte is embedded in the plates.
And that leaves more water in the battery.
What Happens When a Battery Freezes?
The same as what happens when you make ice cubes in a tray.
In both cases, the frozen water expands and pushes against the containment structure.
In a lead-acid battery, this action pushes the lead plates together.
And this can cause a short between the positive and negative plates.
As a result, the battery can lose its structural integrity and thus its ability to effectively produce electrical current.
Can AGM Batteries Freeze?
It’s not uncommon to hear the question, “do AGM batteries freeze?”
The answer?
Yes, any AGM battery can freeze in cold weather.
The AGM battery freezing temperature is generally no different from other batteries’ freezing temperatures.
Can Golf Cart Batteries Freeze?
Yes, like all other batteries, golf cart batteries can freeze.
Because batteries can freeze, especially when discharged, the key to protecting any battery from freezing is to keep it fully charged throughout the cold season.
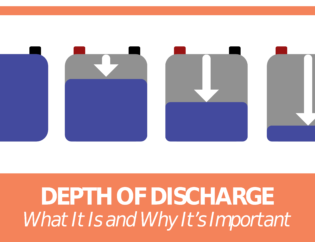
2. You Can Lose Battery Capacity
Besides the potential for freezing, cold weather has a direct impact on battery capacity.
How exactly?
A lead-acid battery’s capacity goes down as the temperature decreases.
And it goes up as the temperature increases.
How much?
The rule of thumb for battery capacity is that for every 15° to 20° below 80° F, the battery loses 10% of its capacity.
Thus, you’ll have less available power from a cold battery than one that is at or above room temperature.
3. You Need a Higher Voltage to Charge
If you’re wondering what the effect of temperature on battery voltage is, here’s the deal...
When charging a lead-acid battery at low temperatures, a higher charge voltage is required than at higher temperatures.
This is because cold temperatures cause the electrolyte to become gel-like.
And that increases resistance in a battery’s chemistry.
As a result, it slows down the electrochemical reaction.
This is why, to improve battery life, charging needs to be “temperature compensated.”
This is especially the case when the battery’s temperature is expected to be below 50° F.
4. The Battery Has a Lower Self-Discharge Rate
Temperature also affects battery charge and discharge rates.
How?
At room temperature (68° F), a lead-acid battery’s self-discharge rate is usually about 3% per month.
But, at low temperatures, a battery’s self-discharge is nearly negligible.
The opposite is also true.
At higher ambient temperatures, a battery’s self-discharge increases.
So in effect, a battery loses less of its energy when it’s cold than when it’s hot.
This is your “how to store a battery” checklist.
Let’s review our top tips for how to keep batteries fresh during the winter.
The following factors will guide you on how to store a lead-acid battery:
Where Should Batteries Be Stored?
Storing lead-acid batteries requires some foresight.
The best way to store batteries is to store them in a clean, dry, cool, and frost-free location away from direct sunlight.

If batteries are exposed to sunlight, they’ll be exposed to excessive temperature.
If that happens, the battery will stop working, bubble, and bulge.
Also, extreme heat from the sun can cause battery corrosion.
And this can shorten the battery’s life.
Can Batteries Be Stored in the Cold?
Answer: No, it’s not advisable to store batteries in the cold.
As we’ve covered, cold temperatures can cause the battery to lose capacity, can freeze the electrolyte, and can result in your battery not working as well as it should.
But the heart of this question is really: “Do batteries last longer in the cold?”

And does freezing batteries extend their life?
The answer to both questions is “No.”
While the self-discharge rate will slow down in the cold, it doesn’t mean the battery will last any longer.
So, what is the proper battery storage temperature?
Try to keep it around 59° F.
Does Concrete Drain Batteries?
The idea that concrete will drain your battery and kill it, and so you should not store your battery on the floor is just a myth.
A battery will drain (called “self-discharging”) no matter what surface you store it on.
So, whether it’s stored on wood, metal, or any other surface, the battery will drain.
But concrete won’t make it drain any more or less than other surfaces.
Are you wondering which battery terminal to disconnect for storage?
Answer: The negative (black) terminal.
That way, you can prevent any electrical loads from draining the battery.
This is because batteries experience parasitic “vampire” loads.
These are small power draws that happen even if the appliance being powered is “off.”
And when combined with the fact that the battery self-discharges, this can weaken the battery.
You should clean the battery prior to storage to remove any corrosion or electrolyte deposits.
That’s because batteries with corrosion, electrolyte deposits, or dirt/dust tend to self-discharge quickly.

Why?
Because they experience transient power loss between the positive and negative terminals.
So, clean the battery terminals with a battery spray cleaner or a baking soda/water mix — as if you’re neutralizing it.
Also, clean the case battery according to your battery manufacturer’s instructions.
Before storing, make sure to charge the battery to a 100% state of charge (SOC) one final time.
Also, ensure that the battery water level is just above the internal plates.
And once stored, periodically check and test the battery’s voltage and specific gravity.
Then, apply a charge when the battery’s SOC falls to 70% or below.
Note: Since lead-acid batteries can have different readings, it’s best to apply the charge based on the manufacturer’s instruction.
Check the manual and confirm because some manufacturers can allow lead-acid batteries to drop up to 60% SOC before recharge.
Now, what are the battery charging guidelines you should follow?
Let’s cover that below.
Use a Winter Battery Charger
The battery charger you use is very important.
You can use a battery maintainer or trickle charger to keep your battery charged while in winter storage.
Battery Maintainers vs. Trickle Chargers: What’s the Difference?
Both of these chargers deliver consistent low-level charges to batteries.
But they aren’t exactly the same.
Here’s the difference:
A trickle charger delivers a charge that is equal to the battery’s self-discharge rate.
Furthermore, this type of charger should be disconnected when it’s done charging.
Otherwise, you risk overcharging the battery.
On the other hand, battery maintainers (also called “float chargers”) are meant to stay plugged in.
Can You Leave a Battery Maintainer on All the Time?
Yes, you can.
The great thing about maintainers is that they’re smart and fully automatic.
So, you can leave them plugged in for a long time.
A battery maintainer’s system is designed to apply the appropriate amount of electric current based on the battery’s charge level.
After the battery is 100% fully charged, the maintainer switches over to float mode.
This way, the maintainer only charges the battery when it drops below a pre-set voltage.
Can You Trickle Charge a Frozen Battery?
No.
Never try to charge, trickle charge, or jumpstart a frozen battery.
Doing so can damage the battery and even cause it to explode!
If you want to use a battery that’s been stored for a long time, first perform a visual check on the case.
When you do this, you’re looking for cracks.

And if there are cracks, the battery is likely too damaged to continue working.
But if there are no cracks and the battery case looks fine, take it to a warm place to unthaw.
Once the battery is unthawed, see if it holds a charge.
If not, you can try charging it or take it to a battery professional to inspect and test it.
How to Store a Lead-Acid Battery: A Summary
Check the battery for external damage (cracks, leaks, etc.)
Charge the battery to 100%
Disconnect the negative (black) cable
Check the battery’s specific gravity or voltage and ensure they’re correct
Ensure there’s enough fluid (for flooded batteries) and top off if necessary
Clean the battery using a baking soda and water paste
Store the battery in a cool (59° F), dry place where it can’t freeze
To make charging easy, store batteries in an easily accessible location
Confirm that you have the right charger for the battery
In storage, periodically check and test the battery’s voltage
Apply a charge when the battery’s charge falls to 70% or below (or whatever manufacturer’s specification is)
Conclusion
There you have it: Everything you need to know about deep cycle battery winter storage.
Knowing this means you can help extend your battery’s shelf life and reduce safety hazards.
Now, we’d like to turn it to you.
Have you run into problems with frozen batteries?
Do you have any further questions?
Please share with us in the comments section!












What temperature is too cold for my flooded deep discharge marine battery? In other words, as I am watching the nighttime temperatures drop in the fall, when is it time to take it out of the boat for the winter?
Hi Johnny,
It’s best to not expose the battery to temperatures below freezing (32° F). If the battery is fully charged, the electrolyte solution should be fully absorbed into the plates, meaning it’s unlikely it will freeze when the temp drops. But if the battery isn’t fully charged, the liquid electrolyte can freeze just like regular water. So to be extra safe, it’s best to keep the battery fully charged and put it into storage before the temp drops to 32°. I hope that helps and thanks for reaching out!
I have battery maintainers on all 4 batteries on my boat. I live in WI, so it gets pretty cold. Based on the information above, it seems that as long as I leave these plugged in and the batteries charged, I should be able to leave my lead acid batteries in the boat, right?
I did have 2 batteries freeze last year because I had to store in a location where they couldn’t be on a charger.
Hi Eric,
Great question. Yes, as long as you maintain the state of charge on the batteries, it should be okay to leave them in your boat. The basic idea is that the higher the state of the charge, the less water is in the battery — unabsorbed from the plates. Thus, with less water, there’s a lower chance that cold temperatures will freeze the battery. I hope that helps!
Comments are closed.