
This article is all about lead-acid battery safety.
In it, you’ll learn:
How lead-acid batteries work
The dangers they present
Proper safety precautions to take when handling batteries
How to clean up battery spills
And much more!
Let’s dive in!
Lead-acid batteries use an electrochemical process to produce energy.
Let’s explain this.
A lead-acid battery consists of metal plates and an electrolyte solution.
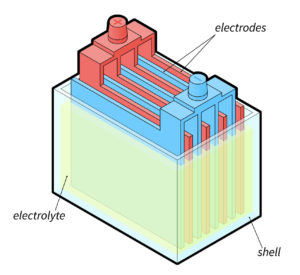
Now, what are the two pieces of different metals that are in contact with electrolytes in a battery?
These 2 metals are:
Lead peroxide (PbO2), which is the positive terminal
Sponge lead (Pb), which is the negative terminal
The electrolyte solution reacts with these 2 metals in order to generate energy.
What Is the Electrolyte Substance in a Lead-Acid Battery?
The electrolyte substance is simply the battery acid.

But what is battery acid?
It’s a fluid that is composed of:
30% to 50% sulfuric acid
Distilled water
So why do batteries have acid?
Because it helps facilitate the conversion of chemical energy into electrical energy.
Let’s go into more detail to find out how exactly that works.
How a Lead-Acid Battery Produces Electricity
So, how do these components work together to produce electricity?
Here’s how:
The two lead plates are immersed in the electrolyte solution
When a load is applied to the battery, electrical ions flow from the sulfuric acid to the negative plate
The movement of the ions produces electricity, which is transferred to the electrical device connected to the battery
The electrical current then leaves the device and moves back into the battery through the positive terminal, where it’s absorbed by the positive plate
When the battery is charged, the opposite action takes place.
That is, the battery converts the charger’s electricity into chemical energy, which is stored inside the battery.
Acid Contact
How Dangerous Is Battery Acid?
Sulfuric acid - the acid in batteries - is an inherently dangerous substance.
In people, battery acid dangers include:
- Burns
- Poisoning
- Blindness
- Internal organ damage
Does Battery Acid Burn?
Yes, it does.

Exposure to battery acid is corrosive to all body tissues and can cause serious injuries or even death in extreme cases.
The Effects of Battery Acid on Skin
What Happens If You Touch Battery Acid?
Any battery acid exposure to tissue can cause chemical burns.
But they might not show up immediately.
You may only realize the burns after several minutes or hours.
Symptoms of chemical burns on the skin can include:
- Dermatitis (red, inflamed skin)
- Necrosis (blackened or dead skin)
- Pain
If you get battery acid or fumes in your eye, it can cause:
- Tearing
- Irritation
- Redness
- Inflammation
- Or even blindness in severe cases
We’ll cover what to do if you get battery acid on your skin in the section called “What Neutralizes Battery Acid on Skin?”
Is Car Battery Corrosion Dangerous to Touch?
The corrosion you may see on a battery is called “sulfation.”
And sulfation is composed of lead sulfate, which is a byproduct of the normal operation of a battery.

As such, it’s composed of potentially dangerous chemicals.
And so you should avoid touching it with your bare hands.
If you do, make sure to wash it off right away.
And ensure you don’t touch your mouth, nose, or eyes in the meantime.
Is Battery Acid Poisonous?
Yes, it is.
The sulfuric acid in battery acid can cause poisoning if swallowed.
Symptoms of swallowing sulfuric acid can include:
- Throat swelling
- Burns in the mouth and throat
- Pain in the mouth and throat
Throat swelling can lead to breathing difficulty, speech problems, and vomiting with blood.
Additionally, the acid can cause serious injuries to your internal organs.
So if you swallow sulfuric acid, seek medical attention immediately.
What Happens If You Inhale Battery Acid?
Inhaling battery acid can irritate the nose and throat and cause respiratory damage.

Immediate symptoms from inhaling battery acid can include:
- Choking
- Rapid pulse
- Shortness of breath
- Dizziness
Later on, you may cough up blood, experience body weakness, bluish fingernails, lips and skin, and chest pains.
If you do inhale battery acid, seek medical attention immediately.
Inhaling Battery Acid Dust: What Can Happen?
Battery dust can be as dangerous as battery acid.
Inhaling it can cause:
- Damage to your mucous membranes
- Severe lung damage
- Adult respiratory distress syndrome
If acid dust is inhaled, take the victim to an area with fresh air.
Then, keep them at rest in a position comfortable for breathing.
And if the victim develops breathing difficulty, obtain immediate medical attention.
Fume Inhalation
Do Lead-Acid Batteries Emit Fumes?
Yes, lead-acid batteries emit hydrogen and oxygen gases during charging.

This gas is colorless, flammable, poisonous, and its odor is similar to rotten eggs.
It’s also heavier than air, which can cause it to accumulate at the bottom of a poorly ventilated space.
Is Battery Gas Harmful?
Yes, battery fumes are harmful.
If inhaled, lead-acid battery fumes can cause damage to the respiratory system or even death at high levels of concentration.
Is Battery Acid Flammable?
Battery acid itself is not flammable.
But the hydrogen gases that it emits during charging are flammable and highly explosive at high concentrations.
Can Battery Acid Start a Fire?
Yes, lead-acid battery fires are possible - though not because of the battery acid itself.
Overall, the National Fire Protection Association says that lead-acid batteries present a low fire hazard.
Furthermore, the NFPA reports that (based on limited information) flooded lead-acid batteries are less prone to thermal runaways than valve-regulated lead-acid batteries (VRLA).
That’s because the liquid solution in flooded batteries can inhibit fire better than the materials inside VRLA batteries can.
What Causes a Lead-Acid Battery to Explode?
The electrolyte’s chemical reaction between the lead plates produces hydrogen and oxygen gases when charging a lead-acid battery.
In a vented lead-acid battery, these gases escape the battery case and relieve excessive pressure.
But when there’s no vent, these gasses build up and concentrate in the battery case.
Since hydrogen is highly explosive, there’s a fire and explosion risk if it builds up to dangerous levels.
What Is a Dangerous Level?
Any hydrogen concentration above 4% is dangerous and explosive.
To reduce the risk of explosion, keep the concentration of hydrogen in the air below 1%.
This is why it’s important to provide plenty of space for the hydrogen to dissipate while charging.

Additionally, you should keep away ignition sources like sparks, high temperatures, and open flames.
This is not only a common-sense safety procedure but it’s also required by OSHA.
Can a Frozen Battery Explode?
Yes, it can.
The freezing of battery electrolyte often occurs when the battery is undercharged and exposed to low temperatures.
This is because when a battery is fully charged, the water and acid in the electrolyte are combined.
And that means it has a very low freezing point.

But if the battery is not fully charged, the water and sulfuric acid will separate.
And this can cause the battery to freeze.
If you try to charge or jumpstart the battery in a frozen state, it can explode.
So, never charge a frozen battery.
Instead, replace the battery to prevent injury or damage.
Electric Shock
Can a Lead-Acid Battery Shock You?
A normal 12-volt lead-acid battery cannot electrocute you if you touch both the positive and negative terminals with your hands at the same time.
Why?
Because the human skin can resist the penetration of 12-volts of electricity.
However, larger industrial lead-acid batteries - like forklift batteries - can potentially electrocute you.

That’s because these batteries often operate on 36-volt, 48-volt, or even up to 80-volt power.
And these higher voltages pose a greater risk for electric shock and possible electrocution.
But in either case, it’s important to never touch both terminals with any conductive materials, like a wrench.
Doing so can cause arcing, which can result in severe burns.
Crushing
A typical 12-volt battery weighs about 40 lbs.
If you drop one on your foot, for instance, you can potentially break bones.
On the other hand, industrial lead-acid batteries can weigh 2,000 lbs. or more.

So if you drop one on yourself, you can cause extremely severe injuries or even death.
So, when handling batteries, you need to take precautions to prevent harming yourself due to the weight.
One way is to always use proper lifting techniques.
This includes:
- Getting your body as close as possible to the battery
- Lifting with your knees
- Or getting a coworker to help you lift it
For industrial-sized batteries, use approved battery handling equipment like a crane or another forklift.
Lead Exposure
As explained in the first section, batteries have lead plates in order to produce electricity.
And it’s been long known that lead can cause serious health issues.
Let’s go through some of the risks of lead when working with batteries.
Can You Get Lead Poisoning from Batteries?
It’s possible to get lead poisoning from batteries due to lead exposure.
Lead exposure often occurs if you work with batteries a lot.
Particularly, any activities like heating, soldering, or sanding lead can release inhalable lead dust particles.

And you can be exposed to lead poisoning by breathing in these dust particles or fumes.
Especially worrying is that lead fumes and dust don’t have any odor.
So, you may never know you’re being exposed.
That’s why you should always wear proper protective equipment like face masks when working with lead-acid batteries.
Is Lead Dangerous to Touch?
Touching lead isn’t itself dangerous.
But when you breathe in, swallow, or otherwise allow the lead to absorb into your body, you can have serious problems.
Therefore, you should always wear gloves when there’s a potential to touch lead.

And if you do touch any of the lead in a battery (or the lead sulfate crystals), you should never touch your eyes, nose, or mouth.
Also, make sure to wash your hands thoroughly to avoid contamination.
Is Lead Flammable?
No, lead isn’t flammable.
This is because of its relatively low melting point (621 °F) and low reactivity with oxygen.
However, since lead-acid batteries can still catch fire due to vented hydrogen gas, you can get hurt from inhaling smoke containing lead.
Get Battery Safety Training
Many online training courses are available to provide lead-acid battery safety training.
Examples of these courses include Conger Industries’ Forklift Operator Training Course and the OSHA Education Center’s Battery and Charger Safety Certificate Course.
When you sign up for such a course, you’ll learn topics like:
How batteries and chargers work
How to properly use batteries
Basic battery safety
How to handle, recharge, maintain, water, and clean batteries
How to clean battery acid spills
How to avoid and manage potential battery handling hazards, such as chemical burns, corrosion, lead poisoning, and electric shock
Battery safety training is ideal for individuals who work with battery-powered equipment, including forklifts, lift trucks, electric buses, and golf carts.
Fortunately for you, if you decide to sign up for one of these courses, you’ll have a solid background already just by reading this article!
Wear the Proper Personal Protective Equipment (PPE)
Wondering what protection is to be worn when working around batteries?

OSHA says it must include:
- Face shield: This will protect your face from the acidic electrolyte
- Apron: This will protect your clothes and body from acid corrosion
- Rubber gloves: These provide both acid protection and electrical resistance to prevent shock
You might also be wondering “will battery acid eat through rubber?”
And the answer is no.
Rubber gloves and aprons are made to withstand leaking battery acid or acid splashes.
So stick to this material to keep yourself safe.
Don’t Mix Metal with Batteries
First things first: You should remove all metallic jewelry before working around batteries.
Why is it important to remove all jewelry before working around batteries?

Because conductive materials like metal can cause a short circuit when coming into contact with a lead-acid battery.
So you should keep all metallic materials away from batteries.
In fact, in standard 1917.157(l), OSHA states that:
“Metallic objects shall not be placed on uncovered batteries.”
Have the Right Safety Equipment Available
In standard 1926.441 - Batteries and battery charging, OSHA states that the required safety equipment when working with batteries should include:
- Eye and body wash station: This will help wash off acid in case of contact
- Fire protection: This will come in handy in case of a fire or explosion
- Neutralizing solution: You will use this material in case of acid spills on the surface, floor, or skin
What Gas Is Produced When Charging a Lead-Acid Battery?
As we’ve discussed, when a battery is being charged, it produces oxygen and hydrogen.
This is a result of the chemical reaction that occurs wherein the electrical current splits the water into its main elements.
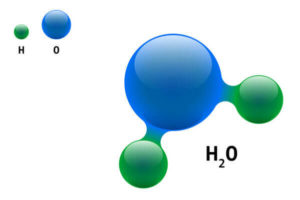
This is why it’s so important to charge the battery in a well-ventilated area.
That way, you can lower the risk of accumulating a dangerous amount of hydrogen gas.
When Does a Battery Give Off the Most Gas?
Most of the gas given off during battery charging happens during the last hour.
How to Safely Charge a Lead-Acid Battery Step-By-Step
If you want to charge a lead-acid forklift battery safely, use the following step-by-step battery charging safety procedure:
-
Park the truck in the charging area and set the parking brake
-
Make sure to turn off the ignition
-
Raise the lift truck’s (material’s) hood. This is to help in ventilation and heat dispersion
-
Disconnect the battery connector from the truck connector
-
Make sure the battery charger is turned off before connecting the battery to the charger
-
Check if the battery’s voltage and amps match that of the charger. You must use the right charger for the battery
-
Inspect the connectors and cables for damage. If you find damage, correct it before proceeding
-
Connect the charger connector to the battery connector (NOT the truck connector!)
-
The battery charger should automatically turn on
-
Once the charger is on, the battery will begin charging
-
When the battery is charging, make sure the vent caps are in place to avoid electrolyte splash or spray
-
Allow the battery to charge up to 100% charge. This can be up to 8 hours, depending on the battery type
-
Once the charging completes, press the charger’s “Stop” button to terminate the charge. If you have an automatic charger, it may automatically stop charging the battery when it reaches 100% charge
-
Disconnect both the connectors
Make sure to allow the battery to cool before using it again.
In most cases, lead-acid batteries need 8 hours to cool.
Why Do Batteries Need to Have Water Added?
Water is part of the electrolyte fluid, which helps in generating power.
Also, water protects the battery’s active material (i.e. lead plates) while it generates power.
So, without water, the battery’s active material may oxidize.
And that will cause the battery to lose power.
Should You Add Water to a Battery Before or After Charging?
Add water to a battery after charging.

Adding water before charging isn’t a good idea because the water may expand during charging.
And this can cause the electrolyte to boil over and spill out.
How to Safely Add Water to a Battery
You should abide by the following safety tips to reduce the risk of injury when adding water to a battery:
Wear appropriate safety PPE
Only add water to the battery. Do NOT add acid
Only water in a designated area. This area should have a stable and level surface, be well-ventilated area, and have no sparks or open flames
Alert any bystander to the battery watering activity so that they take appropriate precautions
Add water until the electrolyte level is just above the lead plates
After watering the battery, rinse off your gloves before removing them and wash your hands with soap and water
Answer: Soap and warm water.
Here’s what to do if you touch battery acid:
Flush your hand (or the area) with lukewarm water and soap.

And if after 30 minutes you still feel a sting or burning, seek immediate medical attention.
What If You Get Battery Acid in Your Eyes?
If acid gets in your eye, flush your eyes with warm water immediately for around 20 to 30 minutes.

Then seek immediate medical help to avoid serious consequences.
What If You Get Battery Acid on Clothing?
Lead-acid battery leakage can corrode your clothes or other equipment within its reach.
So if you get battery acid on your clothing, you should remove it right away.
Otherwise, the acid may eat through the fabric and make contact with your skin.
Once you remove the clothes, you can use a mixture of baking soda and water to neutralize the acid.
Hopefully, this will prevent ruining your clothes.
How to Neutralize a Battery Acid Spill on the Ground
If you spill battery acid on the ground, follow this procedure to clean it up:
-
Mix baking soda with water (3:1 ratio) to form a paste
-
Pour the paste on the acid
-
Wipe the paste (and acid) mixture using a disposable cloth
-
Rinse the spill area with clean water
How to Neutralize Battery Acid on Concrete
Follow the steps above, but apply a generous amount of baking soda paste to the stain using a paint scraper.

Also, use a wire brush to apply the paste to the concrete.
Then, use plenty of clean water to wash the area.
Conclusion
That’s it: The complete guide to lead-acid battery safety.
Now, we’d like to hear from you.
Do you work with lead-acid batteries?
What did you learn about the dangers of lead-acid batteries?
Please share with us in the comments section!








You said charging emits hydrogen sulfide. Then you said charging emits hydrogen and oxygen. Please clarify.
Hi Kurt,
Great catch! That indeed was a mistake. The correct answer is that charging lead-acid batteries produces hydrogen and oxygen gases, due to electricity splitting the water atoms present in the electrolyte solution. Charging does not normally produce hydrogen sulfide. That said, hydrogen sulfide may be present in and/or around the batteries because of the electrolyte, which often contains sulfuric acid.
Comments are closed.